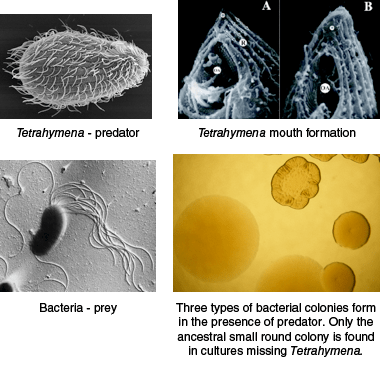
The microevolution lab provides a rare opportunity for students to address evolution and natural selection with a hands-on experiment that can be completed in less than two weeks. The experiment utilizes Pseudomonas fluorescens, a common, nonpathogenic saprophyte that colonizes soil, water and plant surface environments and Tetrahymena thermophila to demonstrate diversifying selection in response to predator-prey interaction. The basic lab can be expanded to compare resource competition and predation as driving forces behind adaptive radiation. Students observe predator-driven real time evolution in a micro-environment in about a week. In the presence of a Tetrahymena predator, clear phenotypic changes in bacterial growth pattern and niche formation are observed in liquid culture, and related changes in bacterial colony formation on agar plates are easily distinguished. Colony formation is dependent on what niche bacteria occupy in the liquid culture, e.g. surface biofilm compared to bottom dwellers.
Module Protocols
Relevant Concepts
Mechanisms of Evolution; Population dynamics; Diversity of Organisms; Mutation;
Prokaryotic and eukaryotic cells; Interdependence in Nature; Continuity and Change.
Next Generation Science Standards Relationships
High School
HS-LS1-3 Plan and conduct an investigation to provide evidence that feedback mechanisms maintain homeostasis.
HS-LS2-6 Evaluate the claims, evidence, and reasoning that the complex interactions in ecosystems maintain relatively consistent numbers and types of organisms in stable conditions, but changing conditions may result in a new ecosystem.
HS-LS3-2 Make and defend a claim based on evidence that inheritable genetic variations may result from: (1) new genetic combinations through meiosis, (2) viable errors occurring during replication, and/or (3) mutations caused by environmental factors.
HS-LS4-1 Communicate scientific information that common ancestry and biological evolution are supported by multiple lines of empirical evidence.
HS-LS4-2 Construct an explanation based on evidence that the process of evolution primarily results from four factors: (1) the potential for a species to increase in number, (2) the heritable genetic variation of individuals in a species due to mutation and sexual reproduction, (3) competition for limited resources, and (4) the proliferation of those organisms that are better able to survive and reproduce in the environment.
HS-LS4-3 Apply concepts of statistics and probability to support explanations that organisms with an advantageous heritable trait tend to increase in proportion to organisms lacking this trait.
HS-LS4-4 Construct an explanation based on evidence for how natural selection leads to adaptation of populations.
HS-LS4-5 Evaluate the evidence supporting claims that changes in environmental conditions may result in: (1) increases in the number of individuals of some species, (2) the emergence of new species over time, and (3) the extinction of other species.
References
●Bantinaki E, Kassen R, Knight CG, Robinson Z, Spiers AJ, Rainey PB. 2007. Adaptive divergence in experimental populations of Pseudomonas fluorescens. III. Mutational origins of wrinkly spreader diversity. Genetics 176 (1):441-453.
●Goymer P, Kahn SG, Malone JG, Gehrig SM, Spiers AJ, Rainey PB. 2006. Adaptive divergence in experimental populations of Pseudomonas fluorescens. II. Role of the GGDEF regulator WspR in evolution and development of the wrinkly spreader phenotype. Genetics 173 (2):515-526.
●Matz C and Kjelleberg S. 2005. Off the hook–how bacteria survive protozoan grazing. Trends Microbiol. 13 (7):302-307.
●McDonald MJ, Gehrig SM, Meintjes PL, Zhang XX, Rainey PB. 2009. Adaptive divergence in experimental populations of Pseudomonas fluorescens. IV. Genetic constraints guide evolutionary trajectories in a parallel adaptive radiation. Genetics 183 (3):1041-1053.
●Meyer JR and Kassen R. 2007. The effects of competition and predation on diversification in a model adaptive radiation. Nature 446 (7134):432-435.
●Rainey PB and Travisano M. 1998. Adaptive radiation in a heterogeneous environment. Nature 394 (6688):69-72.
●Spiers AJ, Bohannon J, Gehrig SM, Rainey PB. 2003. Biofilm formation at the air-liquid interface by the Pseudomonas fluorescens SBW25 wrinkly spreader requires an acetylated form of cellulose. Mol.Microbiol. 50 (1):15-27.
●Spiers AJ, Kahn SG, Bohannon J, Travisano M, Rainey PB. 2002. Adaptive divergence in experimental populations of Pseudomonas fluorescens. I. Genetic and phenotypic bases of wrinkly spreader fitness. Genetics 161 (1):33-46.
See our glossary for the terms used in the modules.